October 14, 2020 at 3:31 am | Updated October 14, 2020 at 3:31 am | 7 min read
The Vast Applications for Spectroscopy in Plant Research
Spectroscopy is a precise and non-destructive technique that can tell us about several processes going on in plants and trees. After being integrated into small handheld devices, it can provide instant and accurate results in the field, forests, and laboratories. It replaces several conventional methods, which were time-consuming and often needed weeks of estimation in expensive laboratories. By reducing time and costs, spectroscopy has found a place in precision farming. It is worth learning about some of its common applications in agriculture, as well as forestry.
Definition of Spectroscopy
Spectroscopy is a technique that measures the different spectra of light that are absorbed or reflected by any matter. Plant tissues, including leaves, are made up of numerous bio-compounds – such as carbohydrates, sugars, starch, amino acids, proteins, pigments, minerals, etc. – all of which have a different spectral signature.
Since spectroscopy is a very sensitive process, it can detect even molecular or atomic differences in compounds.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Uses of Leaf Spectroscopy
Spectroscopy has a wide range of use on different scales. At large scales, it is used in remote sensing to study vegetation types, deforestation, changes in land-use, etc
At the individual plant scale, there are also numerous uses of leaf spectroscopy.
The spectral signature of leaves is not constant. They vary with species, age, plant condition, and environmental factors since these factors affect the many processes occurring in plants. Some of the important processes spectroscopy sheds light on are covered below.
Pigments Measurement
Of all the compounds in a leaf, the pigments absorb the maximum amount of light. Therefore, pigments have a strong spectral signature. Depending on the colour of the light absorbed and those reflected, it is easy to analyze the pigment makeup of leaves.
Though the colour of the leaves is influenced by the predominant pigment at any given time, other pigments are also present in a leaf; hence, leaves are green when chlorophyll concentrations are at their highest. During leaf senescence (leaf death), due to the age of a crop or during autumn in trees, chlorophyll is broken down and other pigments are expressed when their concentrations are higher than the concentration of chlorophyll; so leaves appear yellow, orange, or brown when they are dying (See Figure 1).
Various indices available in spectrometers can be used to calculate the levels of pigments in leaves, such as
- Normalised Difference Vegetation Index (NDVI)
- Anthocyanin Reflectance Index (ARI)
- Plant Senescence Reflectance Index (PRSI)
- Water Band Index (WBI)
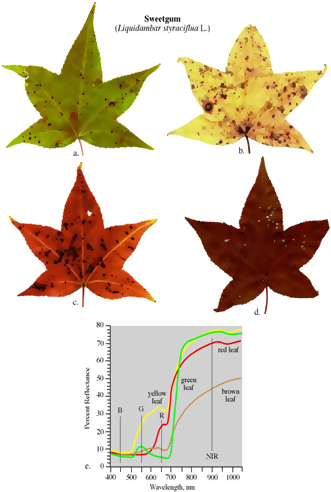
Figure 1: Leaf spectroscopy of Sweetgum Leaves (Liquidambar styraciflua L.) at different ages of the crop. From Jensen 2007. (Image credits http://www.gers.uprm.edu/geol6225/pdfs/08_rs_vegetation.pdf)
Chlorophyll Content in Leaves
Chlorophyll a and chlorophyll b absorb light that is vital to the process of photosynthesis.
Spectroscopy is a non-destructive method to measure the amount of chlorophyll in the leaf canopy of annuals and perennials to predict trends in photosynthesis. Since all compounds produced in the plants start with products of photosynthesis, chlorophyll is very important for plants (See Figure 2).
- Crop productivity: The content of chlorophyll determines how much carbon dioxide a plant takes in and how productivity photosynthesis will be. Thus, monitoring chlorophyll concentrations helps us estimate crop productivity.
- Plant response to agricultural practices: A plant is subject to various stress factors: abiotic, biotic, or even internal physiological processes. The abiotic stress factors are temperature, humidity, mineral nutrition levels, light quality, and herbicides. A change in the production of chlorophyll is one of the most common symptoms of stress. So, by monitoring chlorophyll, it is possible to determine the effect of
- Fertiliser application on crop yield.
- Herbicides on weed health and, therefore, indirectly on crop productivity.
- Stress and different growing conditions on individual varieties.
- Plant health: Healthy plants with more chlorophyll absorb more of red (620-700 nm) and blue (400-500 nm) of the visible light spectrum and reflect more infrared light than unhealthy leaves. So, by checking which spectrum is absorbed and which is reflected, it is possible to estimate the health of a plant.
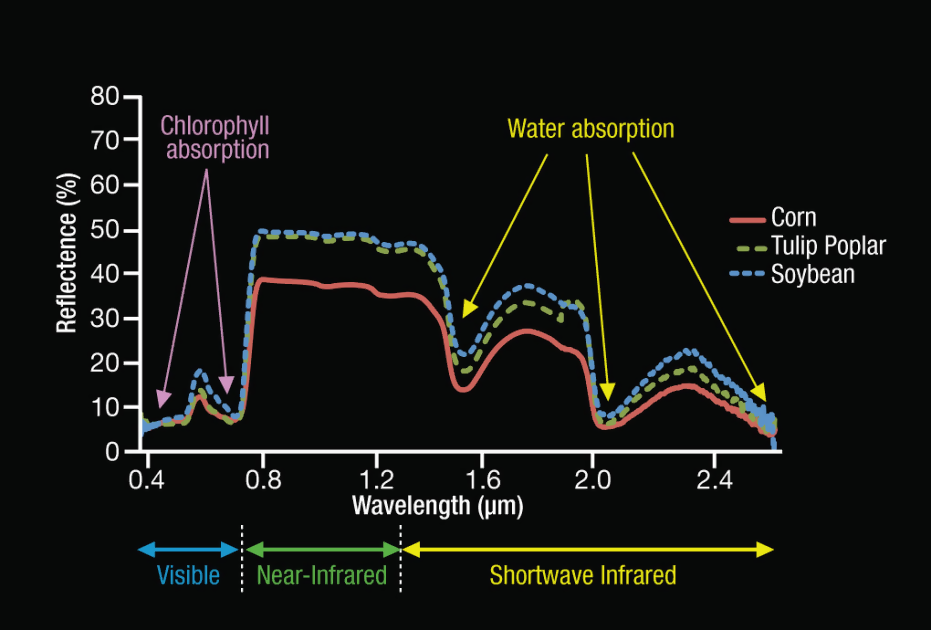
Figure 2: Chlorophyll absorbs light at ~420 and ~650 nm, i.e., blue and red light; water-content in plants absorbs light in the shortwave infrared spectrum. From NASA (Image credits: Eric Brown de Colstoun)
Plant Nutritional Status
It is possible to get a snapshot of the nutrients that plants have absorbed until the day of testing by analysing the plant mineral content through leaf spectroscopy. This method is, in general, better than testing soil nutrient content to detect nutritional imbalances and correct it with proper fertilisation. Then there are other cases where spectroscopy of leaves is particularly suitable as described below.
- Horticultural plants are prone to secondary deficiency, due to problems in plant physiology rather than a lack of the nutrients in the soil. For example, blossom end rot in tomato occurs due to secondary calcium deficiency.
- Yield losses can occur in crops caused by hidden deficiency, which is not expressed, due to slight under fertilisation; these deficiencies are hard to detect as they aren’t expressed in visual symptoms.
- Even over-fertilisation can be harmful due to the toxicity of soil that it produces.
- Spectroscopy is used to estimate N, K, Ca, Mg, B, Fe, Cu, Mn, and Zn concentration. Of them, nitrogen (N) is one of the most commonly monitored minerals in leaves as it is connected to chlorophyll production.
- Leaf spectroscopy helps in detection of hard-to-see deficiencies and improves nutritional management of farms, orchards, and plantations while simultaneously preventing over-use of fertilisers that damage the environment.
Water Content Status
Water content is important for plants, as it is one of the raw ingredients of photosynthesis and is necessary to maintain biomass. Water content in plants can decline due to decreasing soil water content, as well as high temperature, competition among plants, etc.
Therefore, the analysis of plant water content through leaf spectroscopy has many applications besides determining plant productivity (See Figure 3).
It is being used to design water saving strategies and to fight climate change.
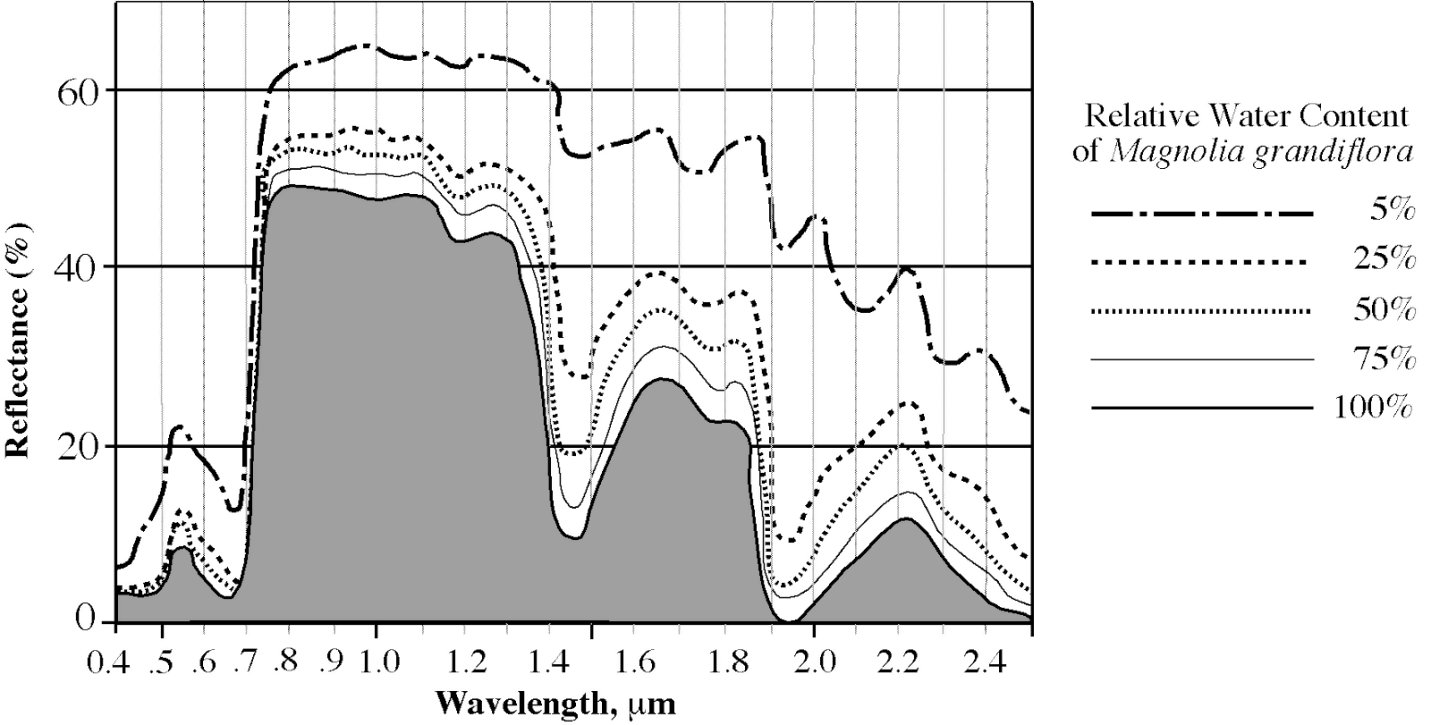
Figure 3: The reflectance changes as the water content in Magnolia (Magnolia grandiflora) decreases. (Image credits: http://cstars.metro.ucdavis.edu/files/3613/4419/0702/Lecture_3-Leaves__Plants.pdf)
Early Detection of Plant Diseases
Plant diseases are a major cause of crop and profit loss for growers. Despite having resistant varieties, it is difficult to eradicate diseases.
Spectroscopy is handy here, as infected leaves have a different spectral image than healthy leaves. The symptoms and stress caused by the pathogens change pigmentation, alter surface temperature, and reduce chlorophyll content.
These changes sometimes begin weeks ahead of the appearance of visual symptoms.
Hence, by monitoring and measuring the change in the spectral image of leaves, early detection of many diseases is possible. The progression of diseases, which is usually accompanied by an increase in severity and development of further symptoms like leaf area loss or lesions, can be arrested with timely and early treatment to reduce crop loss.
Pollution Damages to Leaves
Air pollutants such as sulfuric acid (think acid rain) and ozone cause damage to the leaf surface and roots of plants when they soak into the ground. On the leaves, the pollutants attack and destroy the wax and affect the leaf membrane. The membrane contains chloroplasts, and thylakoids, which, as well as the leaf wax, are bio-chemicals produced by plants.
Alterations in their structure due to pollutants can be detected by spectroscopy to tell us the extent of damage to plants. Depending on the type of damage, it is also possible to identify the pollutant that has caused the leaf damage.
NIR Spectroscopy
Near Infrared (NIR) spectroscopy is one of the most common techniques used in leaf spectrometers. The CI-710 Miniature Leaf Spectrometer, designed and developed by CID Bio-Science, uses NIR spectroscopy to measure transmission, absorption, and reflection of light. It is a small device that is suitable for testing leaves of various sizes and thickness. Its precise and quick results can be immediately used in a wide choice of indices the supporting software provides, making it more versatile.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Sources
Air Pollution and Ecosystems: Proceedings of an International Symposium held in Grenoble, France, 18–22 May 1987. (2013) Edited by Mathy, P. Springer Science & Business Media. Retrieved from https://books.google.de/books?id=ZoLtCAAAQBAJ&dq=Spectroscopy+and+Leaves+-+what+spectrometers+can+tell+us+about+what%27s+going+on+in+plants+and+trees&source=gbs_navlinks_s
Baldacci, L.; Pagano, M.; Masini, L.; Toncelli, A.; Carelli, G.; Storchi, P. Non-invasive absolute measurement of leaf water content using terahertz quantum cascade lasers. Plant Methods 2017, 13, 51.https://doi.org/10.1186/s13007-017-0197-z
Federico Martinelli, Riccardo Scalenghe, Salvatore Davino, Stefano Panno, Giuseppe Scuderi, et al. Advanced methods of plant disease detection. A review. Agronomy for Sustainable Development, Springer Verlag/EDP Sciences/INRA, 2015, 35 (1), pp.1-25. ff10.1007/s13593-014-0246-1ff.ffhal-01284270
Hurst, G. S., Graybeal, J.D., Stoner, J.O., & Chu, S. Spectroscopy. Britannica. Retrieved from https://www.britannica.com/science/spectroscopy
Lecture 3-Leaves. Retrieved from http://cstars.metro.ucdavis.edu/files/3613/4419/0702/Lecture_3-Leaves__Plants.pdf
National Aeronautics and Space Administration, Science Mission Directorate. (2010). Reflected Near-Infrared Waves. Retrieved from http://science.nasa.gov/ems/08_nearinfraredwaves
De Oliveira, L. F. R., De Oliveira, M. L. R., Gomes, F. S, & Santana, R. C. (2017). Estimating foliar nitrogen in Eucalyptus using vegetation indexes. Scientia Agricola, 74, 142-147. https://dx.doi.org/10.1590/1678-992x-2015-0477
Pavlović, D., Nikolić, B., Đurović, S., Waisi, H., Anđelković, A., & Marisavljević, D. (2014). Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomed. (Belgrade), 29, 21–34. DOI: 10.2298/PIF1401021P
Runkle, E. (2017, June). Growing plants with green light. Greenhouse Product News. Retrieved from https://gpnmag.com/article/growing-plants-with-green-light/
The University of Arizona. What is Spectroscopy? Retrieved from http://loke.as.arizona.edu/~ckulesa/camp/spectroscopy_intro.html
Ustin, S.L., & Gamon, J. A. (2010). Remote sensing of plant functional types. New Phytologist 186: 795–816 doi: 10.1111/j.1469-8137.2010.03284.x
Van Maarschalkerweerd, M., & Søren, H. (2015). Recent developments in fast spectroscopy for plant mineral analysis. Front. Plant Sci., &: 169 https://doi.org/10.3389/fpls.2015.00169
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Forest & Plant Canopy Analysis – Tools…
- The Forest Canopy: Structure, Roles & Measurement
- The Importance of Leaf Area Index (LAI) in…
- Root Respiration: Importance and Applications
- Stomatal Conductance: Functions, Measurement, and…
- Irrigating with Saline or Seawater
- Crop Water Use Efficiency Explained



