October 21, 2020 at 9:35 pm | Updated March 16, 2022 at 10:40 am | 8 min read
Developing drought stress tolerance is one of the major targets of many crop breeding programs. Precise and efficient tools to measure drought stress is a vital requirement of these projects. One of the methods widely used by researchers is the measurement of chlorophyll fluorescence. Understanding the concept and the various plant processes that shape it can help in correctly applying this versatile technique in plant science.
Crops’ Exposure to Drought Stress
Crops grown in many parts of the world are being increasingly subjected to drought, which is made worse due to the rise in global temperatures caused by climate change. It is, therefore, necessary for agronomists to breed varieties that are less susceptible to drought and can continue to increase yield to feed a growing population.
Water is a scarce resource; as the area under agriculture is expanded, there is going to be more competition for irrigation. Hence, cultivars that are drought tolerant or resistant and require less watering are going to be an asset.
Subscribe to the CID Bio-Science Weekly article series.
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Crop ecophysiology is the study of the physiological response of crops to the environment. This field of science gives information on plant functions and traits that adapt to changes and stresses.
These traits are the focus of plant breeders who want to increase the yield of crops in heat and drought stress. Chlorophyll fluorescence is one such trait, as it is associated with photosynthesis, which decreases during drought.
What is Chlorophyll Fluorescence?
Only part of the light absorbed by the chlorophyll in the chloroplasts is used to produce carbohydrates. Chlorophyll fluorescence (F) is the one part of the unused light emitted as light; the rest of the unused solar radiation is dissipated as heat; see Figure 1.
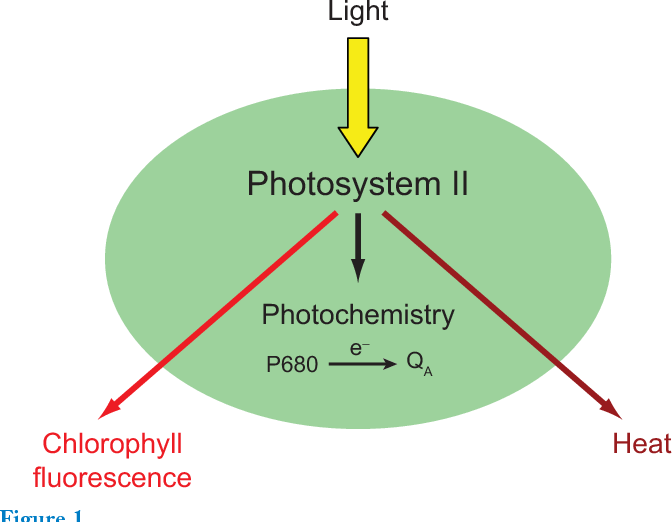
Figure 1: Chlorophyll fluorescence is part of the light not used for photosynthesis by leaves, Baker 2008. (Image credits: DOI:10.1146/annurev.arplant.59.032607.092759)
The color of the light emitted during chlorophyll fluorescence is red or in the far-red wavelengths. Only 1- 2% of the solar light absorbed by the leaf is emitted as chlorophyll fluorescence, but sensitive instruments can measure it.
There are two light dependant steps in photosynthesis: photosystem I and photosystem II (PSII). There is chlorophyll fluorescence in both stages. PSII that is more sensitive to environmental stress, and scientists are interested in changes in its fluorescence.
At the molecular level, chlorophyll fluorescence is all about the movement of photons, or units of light. Photons of light absorbed by leaves are passed along by several molecules of chlorophyll (Chl) a. The photons can be used in photochemistry, lost as heat (non‐radiative decay), or used as fluorescence (emission of a photon).
In photochemistry, the photon is passed on to the reaction center to produce an electron that is then passed along the electron transport chain to split water molecules ultimately.
A change in the flow of electrons indicates an alteration in the efficiency of photosynthesis. When there is less photosynthesis, there is a blockage caused due to unused electrons so that new electrons cannot be produced by light. So, more absorbed solar light is lost, and this can be measured in real time.
PSII photochemistry is also involved in competing processes like photorespiration, electron donation to oxygen, and nitrogen metabolism. Therefore, chlorophyll fluorescence is combined with gas exchange measurements by infrared gas analyzers so that the scientists get a clearer picture of the underlying physiology of plants.
To take into consideration any alterations in ambient carbon dioxide levels due to exhalations during breathing by researchers, a closed leaf system should be used for measurements.
Plant Processes Influencing Chlorophyll Fluorescence
Several processes can influence and change chlorophyll fluorescence during drought. These can be stomatal and non-stomatal.
Stomatal
When a plant experiences drought, the stomata in the leaves, which close to control the rate of transpiration, also restrict carbon dioxide entry into the leaves. As a result, there is less photosynthesis. Stomatal closure is the first event that occurs even in mild leaf drought stress before there is a water deficit in leaves. As drought increases, there is a proportionate closure of stomata and a reduction in photosynthesis. When less light is used, more of it is emitted as chlorophyll fluorescence.
Stomatal closure occurs due to a reduction of leaf turgor, water potential, or air humidity. It is caused by chemical signals, which is the production of abscisic acid (ABA) due to soil drying. Cultivars that are more tolerant of drought close their stomata late in response to drought and allow more photosynthesis. After receiving irrigation, they also open their stomata early, so water use is more efficient. Hence, drought-tolerant varieties can increase yield.
Non-stomatal
Changes in chlorophyll fluorescence, which are non-stomatal, are mainly associated with PS II, which is sensitive to high temperature and drought. These can be photochemical or biochemical processes.
- Photochemical processes- These include a decrease in NADPH and ATP supply, significant steps in photosynthesis. When fewer photons are transmitted due to stomata closing and water hydrolysis decreases, the source of energy and hydrogen ions that change ADP to ATP and NADP+ to NADPH, respectively, also decrease.
- Biochemical processes- Due to water deficit in leaves, there is an increase in the oxygenase activity of RuBisCO. As a result, there is less carbon fixation or photosynthesis.
In short, any factor or process that affects photosynthesis will also change chlorophyll fluorescence.
Chlorophyll Fluorescence Detection Methods
There are two common methods of detecting chlorophyll fluorescence: active and passive.
- In active approaches, the Pulse amplitude-modulated method is commonly used. It is useful for close-range measurements of leaves in the field, greenhouses, or laboratories.
- The passive method, which is used in remote sensing, measures the so‐called solar‐induced Chl fluorescence.
Pulse Amplitude-Modulated

Figure 2: “Typical PAM fluorescence measurement of an Arabidopsis leaf. Fm and Fo are the maximum and minimum fluorescence levels in the dark before actinic light illumination (1000 µmol m−2 s−1, on/off indicated by open arrows). Fs and Fm′ are the steady-state fluorescence and maximum fluorescence levels during actinic light illumination, respectively. Fo′ is the minimum fluorescence level after actinic light is switched off. Fm′ is the maximum fluorescence level following the recovery of the rapidly reversible components of NPQ. Pulses of light (indicated by vertical arrows, 10 000 µmol m−2s−1, normally of 0.5–1.0 s duration) are applied to close all RCIIs and estimate Fm and Fm′.” The image is based on Ruban, 2017. (Image credits: Ruban 2017Quantifying the efficiency of photoprotectionPhil. Trans. R. Soc. B37220160393)
The Pulse Amplitude-Modulated (PAM) method of measuring F is based on the Kautksy Effect. In photosynthesis, there are two phases. The initial phase is the light-dependant phase (also called light reactions). This is followed by the light-independent phase (or dark reactions). It is in the light-dependent phase that light splits water into hydrogen and oxygen, and the excess light is released as heat and fluorescence. In the darkness, this phase stops, so there is no chlorophyll fluorescence either.
When plants are exposed to light after darkness, photosynthesis starts again. The adaption to light is very rapid and for one second the chlorophyll fluorescence peaks. The increase occurs since the electron generated has to be passed on, and until that happens, the PSII cannot absorb more light.
The fluorescence peaks taper down within a few minutes due to two kinds of quenchings, and the leaf soon reaches a steady state.
The peak of fluorescence tapers off, as the speed with which the electrons are passed increases due to the opening of the stomata. This is called photochemical quenching.
The efficiency with which the other part of unused light is converted to heat and removed from the leaf also increases and is called non-photochemical quenching (NPQ). NPQ, or heat dissipation, stops in the dark phase and has to be restarted.
In Pulse-Amplitude-Modulation, chlorophyll fluorometer, a short saturated pulse (SP) of 1μs of low-intensity light, is directed at the leaf. When only a short burst of light is given, NPQ remains zero or negligible, but the fluorescence peak occurs.
This peak, when there is no photochemical quenching yet, is called the maximum fluorescence, Fm. This is compared with the fluorescence at steady state, Ft, and minimum fluorescence Fo during darkness or absence of photosynthetic (actinic) light. Minimum fluorescence is measured using a pulse of measuring light (MP) at the start of the experiment, as shown in Figure 2
The difference between the maximum and minimum fluorescence gives the variable fluorescence Fv. Maximum PSII photochemical efficiency is measured by the ratio Fv/Fm.
The ratio that measures the efficiency of the Photosystem II ΦPSII is:
ΦPSII = (Fm′−Ft)/Fm′, where Fm′ is the maximum fluorescence measured after the saturating pulse.
The CI-340 Handheld Photosynthesis System, manufactured by CID Bio-Science Inc., is a portable tool that measures photosynthesis and stomatal conductance by infrared gas analyzers. The device has a module to measure chlorophyll fluorescence based on the PAM method.
Sun-induced Fluorescence
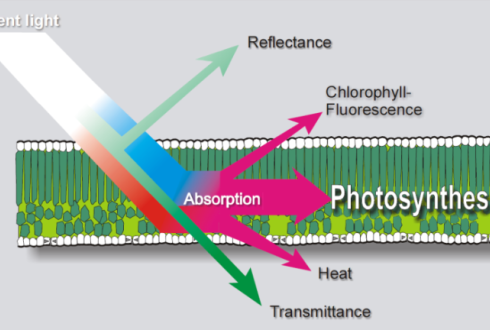
Figure 3: Remote sensing can see the chlorophyll fluorescence of solar radiation, Clevers. (Image credits: https://www.wur.nl/en/article/MSc-thesis-subject-Sun-induced-fluorescence-of-agricultural-crops.htm)
A passive method is used to collect the chlorophyll fluorescence from sunlight and is also called sun-induced fluorescence (F). The data can be obtained by imaging, either by satellites or by proximal remote sensing. The latter method is used in fields; it is rapid and non-invasive, and the sample is placed at a short distance from the imaging sensor to collect reflectance or transmittance, as shown in Figure 3.
Chlorophyll fluorescence lies between the light spectrum range of 650 and 800 nanometres with two peaks at red and far-red. Red is the Fraunhenhofer line of atmospheric oxygen absorption line O2-B (687 nm), and far-red is the oxygen absorption line O2-A (760 nm).
Red fluorescence (F687) is from PS II, while the far-red or 760 nm (F760) is due to PSI. The ratio of red to far-red fluorescence (F687/F760) gives information about environmental stress, based on changes in F687.
Damage to leaves due to drought results in less reabsorption of red light by the leaves. As a result, there is more red light emittance by leaves, and the F687/F 760 ratio increases. This is an easy method to detect drought stress in plants and is widely used in research.
The method is used not only for the drought, but also to detect effects due to pest attacks and other stresses.
A Reliable Method
Chlorophyll fluorescence can be measured in the field or greenhouses by existing portable instruments, such as the CI-340. It is useful in measuring drought stress in annuals and trees. Crop breeding programs rely on this technique to differentiate varieties that are drought tolerant or resistant from those susceptible to water deficiency. Though chlorophyll fluorescence is a small step in photosynthesis among the myriad of processes in plants, its range of applications is remarkable and can also be used for measuring other stresses, too.
—
—
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Feature image courtesy of KoiQuestion
Sources
Baker, N.R. (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual review of plant biology, 59, 89-113. DOI:10.1146/annurev.arplant.59.032607.092759
Brestic, M., & Zivcak, M. (2013). PSII fluorescence techniques for measurement of drought and high temperature stress signal in plants: protocols and applications. In: Rout GR, Das AB (eds.) Molecular stress physiology of plants. Springer Dordrecht pp. 87-131. Retrieved from https://www.researchgate.net/publication/235696104_PSII_Fluorescence_Techniques_for_Measurement_of_Drought_and_High_Temperature_Stress_Signal_in_Crop_Plants_Protocols_and_Applications
Clevers, J. (n.d.). MSc thesis subject: Sun-induced fluorescence of agricultural crops. Wageningen University & Research. https://www.wur.nl/en/article/MSc-thesis-subject-Sun-induced-fluorescence-of-agricultural-crops.htm
Kate Maxwell, Giles N. Johnson, Chlorophyll fluorescence—a practical guide, Journal of Experimental Botany, Volume 51, Issue 345, April 2000, Pages 659–668, https://doi.org/10.1093/jexbot/51.345.659
Koehorst, R.B.M. (n.d.). Pulse-Amplitude-Modulation (PAM). Retrieved from https://www.wur.nl/en/show/PulseAmplitudeModulation.htm
Photosynthesis in Plants. (n.d.). Retrieved from https://photosynthesiseducation.com/photosynthesis-in-plants/
Raji, S.N., Aparna, G.N., Mohanan, C.N., et al. (2017). Proximal Remote Sensing of Herbicide and Drought Stress in Field Grown Colocasia and Sweet Potato Plants by Sunlight-Induced Chlorophyll Fluorescence Imaging. J Indian Soc Remote Sens 45, 463–475. https://doi.org/10.1007/s12524-016-0612-3
Wieneke, S., Burkart, A., Cendrero-Mateo, M., Julitta, T., Rossini, M., Schickling, A., … Rascher, U. (2018). Linking photosynthesis and sun-induced fluorescence at sub-daily to seasonal scales. Remote Sensing of Environment, 219, 247–258. doi: 10.1016/j.rse.2018.10.019
Related Products
Most Popular Articles
- Transpiration in Plants: Its Importance and Applications
- Leaf Area – How & Why Measuring Leaf Area…
- How to Analyze Photosynthesis in Plants: Methods and Tools
- Forest & Plant Canopy Analysis – Tools…
- The Forest Canopy: Structure, Roles & Measurement
- The Importance of Leaf Area Index (LAI) in…
- Root Respiration: Importance and Applications
- Stomatal Conductance: Functions, Measurement, and…
- Irrigating with Saline or Seawater
- Crop Water Use Efficiency Explained




